Corrosion is one of the leading causes of performance degradation and equipment failure in chillers. Whether in HVAC systems, industrial process cooling, or data center cooling infrastructure, corrosion can cause leaks, reduce heat transfer efficiency, and shorten equipment lifespan. Among several factors contributing to corrosion, dissolved oxygen in the coolant plays a critical role.
In this article, we’ll explore how oxygen causes corrosion, why its removal helps, the techniques used to deoxygenate coolant, and additional best practices for chiller corrosion prevention.
1. Understanding Chiller Corrosion
Chillers operate by circulating a coolant — typically water or a water-glycol mixture — through a closed-loop system to transfer heat. However, the presence of oxygen, minerals, and other contaminants can create a corrosive environment, especially when metals such as steel, copper, and aluminum are involved.
Types of corrosion in chillers include:
Uniform corrosion – gradual material loss due to oxidation
Pitting corrosion – localized, deep damage caused by trapped oxygen
Galvanic corrosion – occurs when two dissimilar metals interact in the presence of an electrolyte
Microbiologically Influenced Corrosion (MIC) – caused by bacteria producing corrosive byproducts
Among these, dissolved oxygen accelerates oxidation reactions, making it a primary target for prevention strategies.
2. Role of Oxygen in Coolant Corrosion
Oxygen is a strong oxidizing agent that reacts with metals in the cooling system, forming oxides, rust, and scale deposits. These reactions compromise structural integrity and heat transfer efficiency.
Chemical Reaction Example (Iron Corrosion):
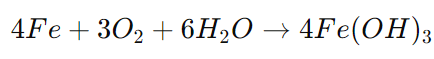
This hydrated iron oxide eventually dehydrates into rust(Fe2O3), which weakens the metal over time.
How Oxygen Accelerates Corrosion
Promotes electrochemical reactions on metal surfaces
Increases the formation of acidic compounds in the coolant
Enhances pitting, especially in stagnant water zones
Accelerates galvanic corrosion when different metals are used
The more oxygen present, the faster the reaction — which is why deoxygenation is a proven corrosion-control method.
3. Does Removing Oxygen Help?
Yes, removing oxygen from the coolant significantly reduces chiller corrosion, especially in closed-loop systems. Lower oxygen levels slow oxidation reactions and minimize pitting, scale formation, and metal deterioration.
Benefits of oxygen removal include:
Reduced corrosion rates — Protects steel, copper, and aluminum components
Extended equipment lifespan — Minimizes premature failure
Improved heat transfer efficiency — Prevents scale buildup
Lower maintenance costs — Reduces repair frequency
Better water chemistry control — Makes inhibitors more effective
However, oxygen removal alone isn’t enough. It should be part of a comprehensive water treatment program that also manages pH, hardness, microbial growth, and corrosion inhibitors.
4. Methods to Remove Oxygen from Coolant
There are several proven techniques to control dissolved oxygen levels in chillers:
A. Mechanical Deaeration
Uses vacuum deaerators or spray-type deaerators
Physically removes oxygen by lowering pressure and increasing water temperature
Common in power plants and large industrial chillers
B. Chemical Oxygen Scavengers
Chemicals react with dissolved oxygen, neutralizing its corrosive effects
Common oxygen scavengers include:
Sodium sulfite
Hydrazine (less common due to toxicity)
Carbohydrazide
DEHA (Diethylhydroxylamine)
Often combined with corrosion inhibitors for maximum protection
C. Vacuum Degassing
Removes oxygen by applying negative pressure
Ideal for closed-loop chilled water systems
D. Membrane Degassing Technology
Uses hydrophobic membranes to separate dissolved gases from the coolant
Highly effective and energy-efficient for modern systems
5. Complementary Strategies for Corrosion Prevention
Oxygen removal works best when combined with other best practices:
pH Control: Keep pH within the recommended range (typically 8.0–9.0)
Use of Corrosion Inhibitors: Protects metals by forming a protective film
Filtration Systems: Removes particulates that can accelerate corrosion
Microbial Control: Prevents biofilm formation and MIC
Regular Water Testing: Ensures stable chemistry and early detection of issues
6. Conclusion
Removing dissolved oxygen from coolant significantly reduces chiller corrosion, improves operational efficiency, and extends equipment lifespan. However, it should not be treated as a standalone solution. A holistic approach — combining oxygen removal, chemical treatment, pH control, and routine monitoring — delivers the best results.
Investing in oxygen control pays off in lower maintenance costs, higher energy efficiency, and better long-term system reliability.
 En
En
 عربى
عربى 中文简体
中文简体

