The so-called limit carbonate hardness (Hj) refers to the critical value at which CaCO₃ does not precipitate under specific water quality conditions and temperatures, where free CO₂ is either absent or minimal. Typically, in cooling water systems, this value ranges from 2 to 4.5 mg equivalent/L. However, by adding acid and scale inhibitors, a cooling water system can maintain higher carbonate hardness levels. This article explains the relationship between scale inhibitors for cooling water and the limit carbonate hardness, providing useful information for water treatment professionals.
1. Acid Addition and Limit Carbonate Hardness
By adding acid to the makeup water, the carbonate hardness is converted into non-carbonate hardness with higher solubility (such as CaSO₄ and CaCl₂), which reduces the carbonate hardness of the circulating water to a level below the limit carbonate hardness, thereby preventing scaling. The chemical reactions are as follows:
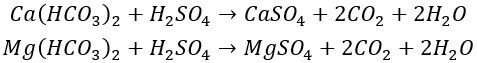
Continue to share a method for calculating the amount of acid to be added based on carbonate hardness and the limit carbonate hardness (Hj), as shown in the following formula.
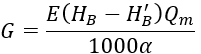
In the formula:
G is the amount of acid added, kg/h;
E is the molar mass of the acid, for sulfuric acid, E = 49, and for hydrochloric acid, E = 36.5;
Qm is the supplementary water volume of the circulating cooling water, m³/h;
α is the concentration of the acid;
HB is the carbonate hardness of the supplementary water, mmol/L;
H′B is the carbonate hardness of the supplementary water after acid treatment, mmol/L.
H′B can be calculated as follows.
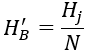
In the formula: N is the concentration multiple; Hj is the limiting carbonate hardness of the circulating cooling water system, in mmol/L.
The limit carbonate hardness of the circulating water after adding acid, without the scale inhibitor, can be calculated using the following formula:
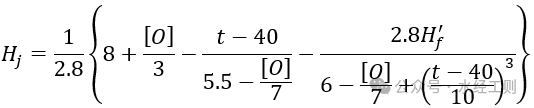
In the formula, [O] represents the oxygen consumption, in mg/L; t represents the temperature of the circulating water, in ℃.
Hf′ is the non-carbonate hardness after treatment with acid added to the supplementary water, in mmol/L, and can be calculated using the following formula:
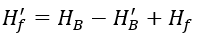
2. Use of Scale Inhibitors with Acid Treatment for Limiting Carbonate Hardness
When using acid treatment for limiting carbonate hardness in conjunction with scale inhibitors, the type of scale inhibitor used should determine the appropriate Hj value. Common scale inhibitors include polyphosphates, organic phosphonates (salts), and polyacrylic acids.
Polyphosphate Scale Inhibitors
Polyphosphates primarily refer to sodium polyphosphate, commonly used forms being sodium hexametaphosphate (also known as sodium polymetaphosphate) and sodium tripolyphosphate. These inhibitors disperse and stabilize colloidal particles and have strong chelation capabilities for calcium and magnesium ions. Sodium polyphosphate not only functions as a scale inhibitor but also has corrosion inhibition properties. The specific properties vary depending on the molecular structure of [NaPO₃]n, where the value of n determines the characteristics. Sodium hexametaphosphate has the chemical formula [NaPO₃]₆ONa₂ and is a polymer of sodium metaphosphate (NaPO₃). When used as a scale inhibitor, the limiting carbonate hardness Hj of the circulating water can be estimated by the following formula. The typical dosage of sodium hexametaphosphate ranges from 1 to 5 mg/L, with the upper limit used for water with high carbonate hardness. Sodium tripolyphosphate (Na₅P₃O₁₀) has a strong ability to chelate calcium ions, with a typical dosage of 2 to 5 mg/L, and Hj = 5 mmol/L.
The drawback of polyphosphates is their tendency to decompose into orthophosphates in water, a process known as polyphosphate hydrolysis. The degree of hydrolysis is influenced by factors such as pH, temperature, time, and microbial activity. Hydrolysis is positively correlated with water temperature and contact time, although it occurs at a relatively slow rate, with typical hydrolysis rates between 11% and 35%.
Organic Phosphonates and Their Salts
These scale inhibitors are effective and also provide corrosion inhibition, making them dual-purpose inhibitors. Many of their properties are similar to polyphosphates, but they are more stable and less prone to hydrolysis, even at higher temperatures. However, organic phosphonates can be corrosive to copper, so they are not suitable for use in copper heat exchanger systems. Common organic phosphonates and their salts used domestically include hydroxyethylidene diphosphonic acid (HEDP), aminotrimethylene phosphonic acid (ATMP), and ethylenediaminetetra(methylenephosphonic acid) (EDTMP). When used together with polyphosphates, these inhibitors can have a synergistic effect, improving the limiting carbonate hardness of circulating water and reducing the dosage of each agent. The typical limiting carbonate hardness for these inhibitors is as follows:
HEDP: Hj = 8 mmol/L
ATMP: Hj = 9 mmol/L
EDTMP: Hj = 8 mmol/L
Polycarboxylate Polymers
Polycarboxylate polymers are polymers containing carboxyl functional groups (carboxyl groups) or derivatives of carboxylic acids. The carboxylate anion (COO⁻) determines the characteristics of these polymers, where M represents a monovalent cation, hydrogen, or an amine group. After being introduced into water, the carboxylate group dissociates into COO⁻ and M⁺, with the COO⁻ being responsible for scale inhibition. Common polycarboxylate scale inhibitors used domestically include polyacrylic acid, sodium polyacrylate, polymethyl methacrylate, copolymers of acrylic acid and hydroxypropyl acrylate, copolymers of acrylic acid and acrylates, and hydrolyzed poly(maleic acid) (anhydride). The typical dosages and corresponding limiting carbonate hardness values are as follows:
Polyacrylic acid: 1–9 mg/L, Hj = 5.5–10 mmol/L
Sodium polyacrylate: 1–8 mg/L, Hj = 5.8–9 mmol/L
Poly(maleic acid): 1–5 mg/L, Hj = 5–8.5 mmol/L
Summary
By controlling the limiting carbonate hardness in circulating cooling water systems, scale formation can be prevented. Using the methods above to calculate the appropriate dosage of acids and scale inhibitors, along with the system's allowable limiting carbonate hardness under specific operating conditions, helps prevent scaling issues while also reducing chemical costs.
 En
En
 عربى
عربى 中文简体
中文简体

